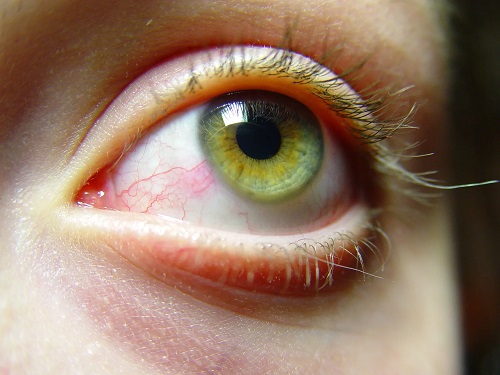
Dry eye syndrome (DES) affects more than 30 million people in the US alone. DES is characterized by ocular surface inflammation. Consequently, patients can suffer from burning, irritation, severe discomfort, foreign body sensation, and blurry and decreased vision. Thymosin beta 4 (Tβ4) significantly improves signs and symptoms of dry eye without any toxic effects.
Current wound healing and anti-inflammatory treatment options for the cornea are limited, and some of the agents used may cause significant complications and side effects. Tβ4 promotes corneal epithelial cell migration, decreases the expression of corneal epithelial cytokines and chemokines (IL-1β and IL-8, respectively), and inhibits corneal PMN infiltration and adhesion to endothelium. Björklund Pharma AS focuses on Tβ4 product development.
PubMed articles: https://bit.ly/3Rw9BxC