Product Candidate
Thymosin Beta 4 in Dermal Healing
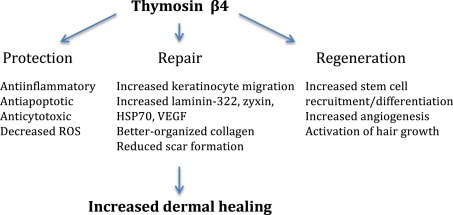
Unlike many pharmacological treatments for various pathologies, much is known about the mechanisms of action of thymosin beta 4 (Tβ4). The active site(s), receptor, signaling pathways, and biological activities are known. The safety profile is excellent, and no preclinical toxicology has been found (1).
Efficacy trends have been observed in phase 2 clinical trials, which reduced the healing time by almost half. Also, a near-optimal dose (0.02–0.03%, w/w) has been identified in dosing studies for dermal healing. Finally, no adverse reactions have been observed with topical or injected Tβ4 in humans, and no autoantibodies have been detected. Tβ4 is a naturally occurring molecule that relatively cheaply can be synthesized. The cost is important given that other treatments are not widely used due to the high cost and limited efficacy (1).
Reference
1. Kleinman HK, Sosne G. Thymosin β4 promotes dermal healing. Vitam Horm 2016;102:251-75. doi: 10.1016/bs.vh.2016.04.005.